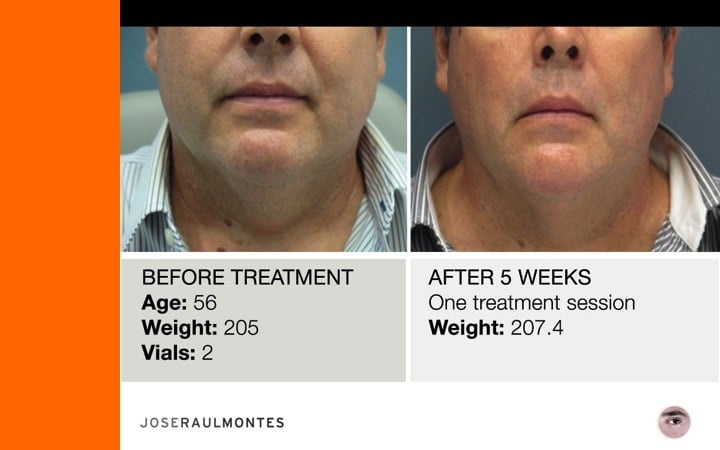
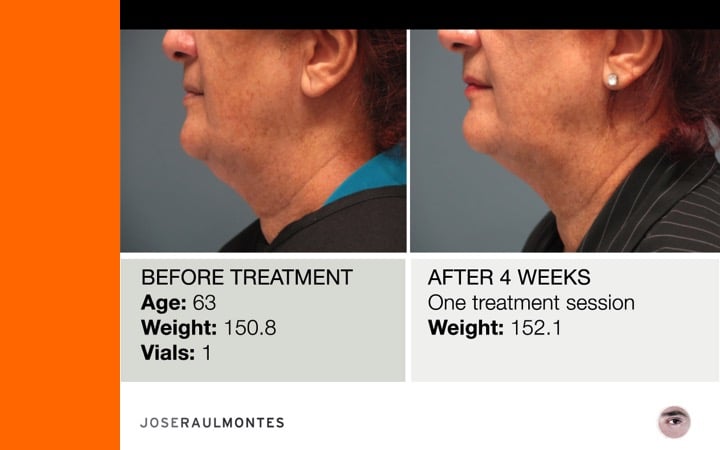
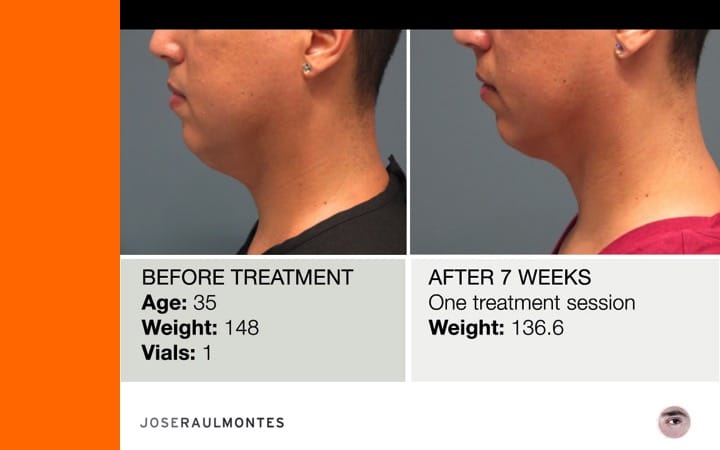
Thinking twice? Call Dr. José Raul Montes now to get your Kybella treatment in Puerto Rico.
Together with Dr. Montes and his staff, discover the best beauty center in San Juan, Puerto Rico.
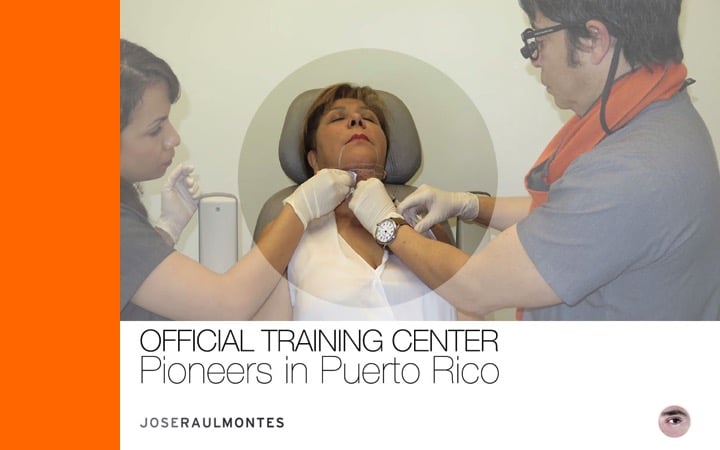
Dr. José Raúl Montes is the Pioneer and Trainer in Puerto Rico of the first FDA-approved treatment for double chin with Kybella!


J.R. Montes Eyes & Facial Rejuvenation was one of the first training sites and among the first injectors.

After two years, over 323 patients have been injected – men & women – from the ages 25 to 79 years, with amazing results!
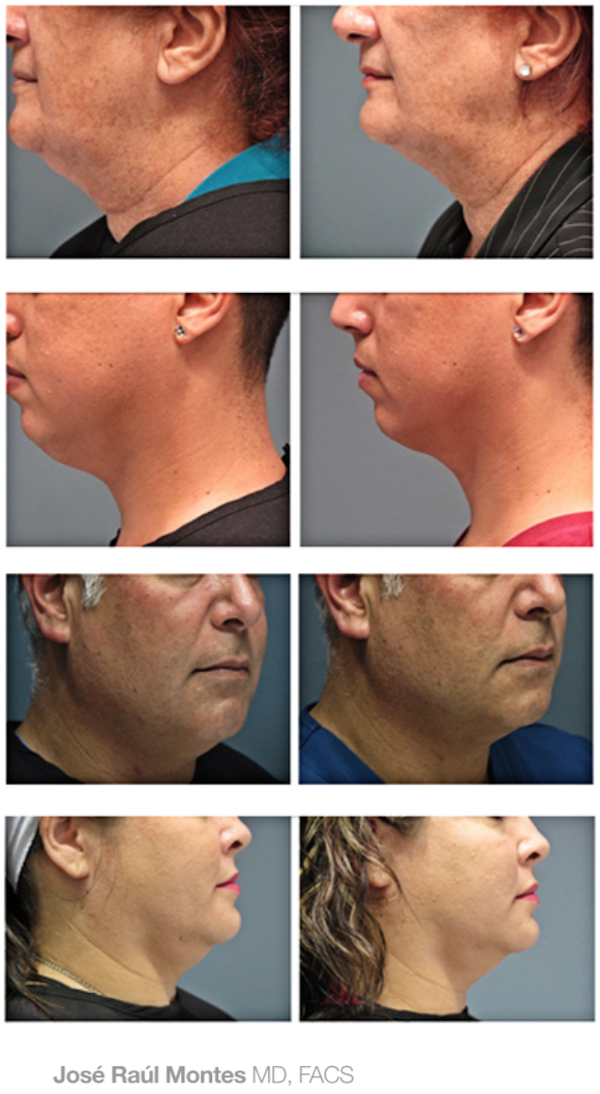

J.R. Montes Eyes & Facial Rejuvenation has been recognized among the Top 20 Best Kybella Practices in the U.S.!

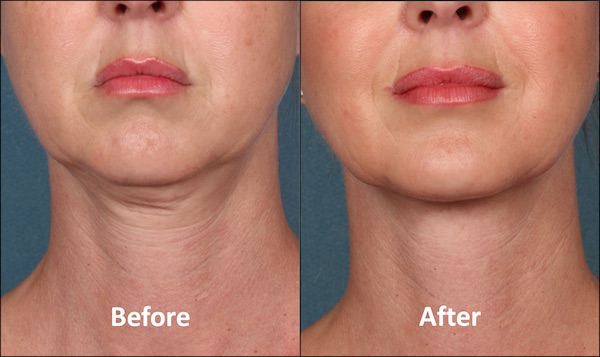
El proceso de envejecimiento y el sobrepeso son de las dos razones principales para la acumulación de grasa en el cuerpo y en especial, en el área submentoniana, mejor conocida como la papada.
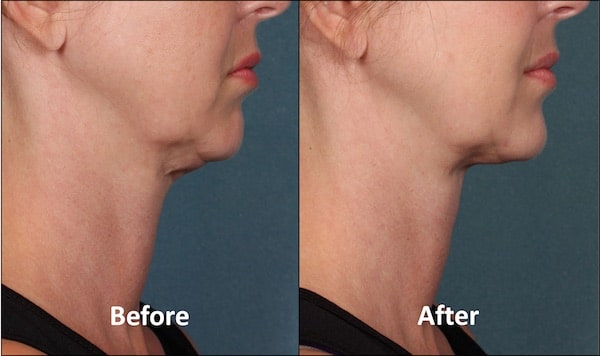
Por años, la liposucción ha sido la opción para deshacerse del exceso de grasa en esta zona, pero en las próximas semanas llegará Kybella a la Isla. El primer tratamiento inyectable dirigido a eliminar la grasa que ha sido aprobado por la Administración de Drogas y Alimentos (FDA).

Kybella es un ácido desoxicólico, que es un ácido que básicamente lo que hace es romper las células de grasa. Se aprobó por la FDA a finales de abril y debe estar disponibles para los pacientes para principios de agosto.
Se trata de un medicamento citolítico, esto es, que, cuando es inyectado en un tejido, destruye su membrana celular. Así, cuando el Kybella se inyecta en la papada, lo que hace es destuir las células de grasas que la forman.
No obstante, desde la FDA advierten: también puede destruir otro tipo de células, como las de la piel, por lo que su administración deberá estar supervisada por un profesional.
Es importante recordar que Kybella sólo se ha aprobado para tratar la grasa de la papada, y que no sabemos si funciona o es seguro para otras partes del cuerpo», declaraba Amy G. Egan, de la FDA.



WAPA TV NotiSalud: Kybella™ (July 1, 2015)
Introducción: Rafael Lenín López
(5 de agosto de 2015)
Ivonne: La innovación científica no se detiene. Puerto Rico es Pionero. Ahora puedes eliminar la incómoda grasa de la papada sin cirugía. El cirujano José Raúl Montes nos acompaña para hablarles de Kybella™. Buenos días. Bienvenido.Dr. JRM: Buenos días Ivonne.
Ivonne: ¿De qué se trata esto de Kybella™?
Dr. JRM: Kybella™ es un producto —se conoce como ácido desoxicólico— es un producto o una sustancia que tenemos naturalmente en nuestros cuerpo y que disuelve la grasa que tenemos. Se ha sintetizado este producto, no es derivado de animal, ni es derivado de personas. Es sintético, así que es muy seguro y se utiliza para la reducción de la grasa debajo del mentón o lo que se conoce como la papada.
Ivonne: ¿Cómo es el tratamiento?
Dr. JRM: Primero que nada —Ivonne— con este producto (como estamos viendo aquí) se marca al paciente, se evalúa al paciente y se protegen todas las estructuras importantes, donde está la glándula de tiroide, donde está la musculatura, etc. y se inyecta específicamente en ese patrón como están viendo en esos puntos —que es donde están localizadas lo que se conoce como la grasa submental— que es lo que forma la papada. El paciente viene a la oficina, ésta es la preparación que requiere: fotografía, marcado, anestesia local y procedimiento que puede durar en la oficina como unos 10 minutos.
Ivonne: Wow.
Dr. JRM: Al cabo de ese procedimiento, el paciente se reintegra a sus funciones normales, eso quiere decir que no tiene tiempo de recuperación. Lo que es interesante de este producto es que fue aprobado por FDA [Food and Drug Administration – Administración de Drogas y Alimentos] en una votación de 17-0. Es decir, todos los miembros de este panel votaron a favor del producto para esta indicación de reducir la grasa submental o la papada. Tenemos encuestas que dicen que alrededor del 70% de las personas están interesadas en este tipo de tratamiento.
Ivonne: ¿Y Puerto Rico es pionero y Estados Unidos? ¿Esta vez los tratamientos no vienen de Europa?
Dr. JRM: Esto es otro de los datos importantes de este producto —de esta aprobación— se aprueba por primera vez en Estados Unidos antes que en el resto del mundo, junto con Puerto Rico, que fuimos escogidos como uno de los centros de entrenamiento y pioneros en el tratamiento.
Ivonne: ¿Cuándo se ve el cambio?
Dr. JRM: El paciente se debe realizar —aquí vemos unos antes y después de los efectos que vamos a ver después de un tratamiento de cómo tres sesiones. En promedio, el paciente va a necesitar de dos a tres sesiones de tratamiento, dependiendo del grado de grasa que tenga o de papada (de la grasa submental). Debemos recalcar —Ivonne— que éste es el primer producto FDA-aprobado para esta indicación en el mundo y que se aprueba por primera vez en Estados Unidos y tenemos la suerte y el orgullo de decir que Puerto Rico también es pionero y nuestra oficina se ha convertido en el centro de entrenamiento para este producto.
Ivonne: Bueno, obviamente esto tiene que ser en un consultorio médico. Esto no un tratamiento que se pueda aplicarse una persona por sí sola.
Dr. JRM: Definitivamente ‘no’ porque tiene que haber un grado bien, bien alto de conocimiento de anatomía de esta área del cuello —de las estructuras vitales para protegerlas— y el tratamiento tiene que ser específico en esta grasa. Se van a hacer entrenamientos en la oficina, solamente a médicos que la compañía escoja, que están más capacitados para usar este producto para que sea exitoso, como ha sido en todos los estudios: alrededor de 2,600 pacientes que ya se han tratado.
Ivonne: Bueno, muchísimas gracias por estar con nosotros y traernos estas noticias.
Dr. JRM: Un placer
Telenoticias Telemundo: Kybella™
(August 5, 2015)
Ivonne: Scientific innovation does not stop. Puerto Rico is among the first. Now you can eliminate the uncomfortable double chin without surgery. Surgeon José Raúl Montes joins us to talk about Kybella ™. Good Morning. Welcome.Dr. JRM: Good morning Ivonne.
Ivonne: What is Kybella™?
Dr. JRM: Kybella™ is a product —a deoxycholic acid— a product or substance which we naturally have in our body and which dissolves fat. This non-animal, non-human derived substance is synthetic, so it is very safe, and it is used to reduce submental fat or what is known as the double chin.
Ivonne: How is the treatment done?
Dr. JRM: First of all —Ivonne— with this product (as seen here) the patient is marked, evaluated and the important structures, thyroid gland and muscles, are protected. It is specifically injected within a pattern as can be seen in those points where the submental fat, known as double chin, is located. This is the preparation required when the patient comes to the office: photography, marking, local anesthesia, and the procedure which can last about 10 minutes in the office.
Ivonne: Wow.
Dr. JRM: After this procedure, the patient can return to his/her normal functions that means no recovery (downtown) is needed. What is interesting about this product is that it was approved by FDA [Food and Drug Administration] by a 17-0 vote. This mean that all members of the panel voted for the product for the indication to reduce submental fat or double chin. There are research studies showing that about 70% of people are interested in this type of treatment.
Ivonne: Puerto Rico and the United states are pioneers? On this occasion this treatment did not come from Europe?
Dr. JRM: That is another important fact about product —of its approval— it is first approved in the US before the rest of the world, and along with Puerto Rico. We were selected as one of the training centers and pioneers of this treatment.
Ivonne: When is the change noticeable?
Dr. JRM: You can see here some before and after visuals of about three treatment sessions. On average, the patient will require two to three treatment sessions, depending on the amount of fat (double chin) he/she has. We must emphasize —Ivonne— that this is the first FDA approved product for this indication in the world, and it was approved for the first time in the United States. We are fortunate and proud to say that Puerto Rico is also a pioneer and our office has become the training center for this product.
Ivonne: Well, obviously this has to be done in a medical office. This is not a treatment that a person can apply at home.
Dr. JRM: Definitely ‘no’ because the person has to have an extremely high knowledge of the anatomy in the neck area —protect vital structures— and the treatment is specifically for this area. There will be workshops at the office only for doctors selected by the company who will use this product successfully, as it has been done with the research studies: about 2,600 patients have already been treated.
Ivonne: Well, thank you for joining us and bringing the news.
Dr. JRM: My pleasure
KYBELLA FACT SHEETS
What is submental fullness?
• Submental fullness due to submental fat, sometimes referred to as ”double chin,” is a common, yet undertreated facial aesthetic condition. It can detract from an otherwise balanced and harmonious facial appearancei – leading to an older and heavier look.
• Submental fullness can impact a broad range of adults, and is not limited to people who are overweight. This condition can impact men or women of average weight and can be caused by aging, genetics and weight gain. It is often resistant to diet and exercise alone.
• According to a 2014 survey by the American Society for Dermatologic Surgery (ASDS), over 2/3 of consumers are bothered by submental fullness – nearly as many as those bothered by lines and wrinkles around the eyes.
What is Kybella (deoxycholic acid) injection?
• Kybella (deoxycholic acid) injection, also known as ATX-101, is the first and only FDA-approved injectable drug that contours and improves the appearance of submental fullness due to submental fat.
• Kybella is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The safe and effective use ofKybella for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended.
How does Kybella work?
• Kybella is a non-human and non-animal formulation of deoxycholic acid, a naturally-occurring molecule in the body that aids in the breakdown and absorption of dietary fat.
• When injected into subcutaneous fat, Kybella causes the destruction of fat cells.iv Once destroyed, those cells cannot store or accumulate fat.v After the aesthetic response is achieved, retreatment with Kybella is not expected. Due to its cytolytic activity, Kybella should not be injected into or in close proximity to vulnerable anatomic structures.
How is Kybella administered?
• Kybella is administered by injections into the fat under the chin.
• Each in-office treatment session is typically 15-20 minutes.
• Treatment with Kybella is customized by the physician to the patient’s aesthetic goals for an improved chin profile.
What are the results of Kybella clinical trials?
• In the pooled, pivotal Phase III studies, 68.2 percent of patients responded to Kybella based on a composite of validated physician and patient measurements.
• Many patients experienced visible results in two to four treatments. Kybella treatment resulted in high patient satisfaction.
• In clinical studies, 28%, 43% and 55% of Kybella-treated patients had a ≥1-grade composite improvement after 2, 3 and 4 treatments,
respectively.
• Patients also reported improvement in the emotional impact of submental fat when asked how happy, bothered, self-conscious, embarrassed, old and overweight they felt following treatment in relation to the amount of their submental fat.
Is Kybella safe?
• Kybella has been the focus of a global clinical development program involving over 20 clinical studies with more than 2,600 patients worldwide, of which over 1,600 have been treated with Kybella.
• Production of Kybella is a highly controlled, quality-assured and validated, current Good Manufacturing Practices-compliant process to ensure patient safety. Kybella contains no human or animal-derived substances.
What are the side effects with Kybella?
• The safety profile of Kybella is well characterized. Side effects may include swelling, bruising, pain, numbness, redness or formation of small areas of firmness. Adverse events with Kybella infrequently resulted in discontinuation from study (1.6% of participants). Care must be taken when injecting Kybella to avoid the risk of marginal mandibular nerve injury and dysphagia.
When will Kybella be available?
• KYTHERA has chosen to execute a training-led launch and developed a training program to educate physicians on the safe use of Kybella, and its approved indication. Physician faculty education will begin in June 2015. Kybella physician training programs will initiate in late summer. Physicians will be able to purchase Kybella and treat their patients after they have been trained.
Kybella should only be administered by a trained healthcare professional.
In Puerto Rico, José Raul Montes Eyes & Facial Rejuvenation is founded on dedication, safety, and excellence.