| BELOTERO BALANCE | JUVÉDERM | RADIESSE | RESTYLANE | RHA COLLECTION DERMAL FILLERS | SCULPTRA® |

Dr. José Raul Montes is chosen as one of the TOP INJECTORS to TRY the products before it was officially launched in the US COSMETICS MARKET!
The RHA® Collection is the newest advancement in HA filler technology, purposefully designed for distinct patient needs.


1. The Science
2. Products
3. RHA® 2
4. RHA® 3
5. RHA® 4
RHA® is Resilient Hyaluronic Acid, and represents the latest advancement in HA filler science: designed for dynamic wrinkles and folds to help you keep your patients looking beautiful at rest, and flawless in motion.
RHA® is the first and only resilient hyaluronic acid filler designed for facial dynamics.




THE SCIENCE
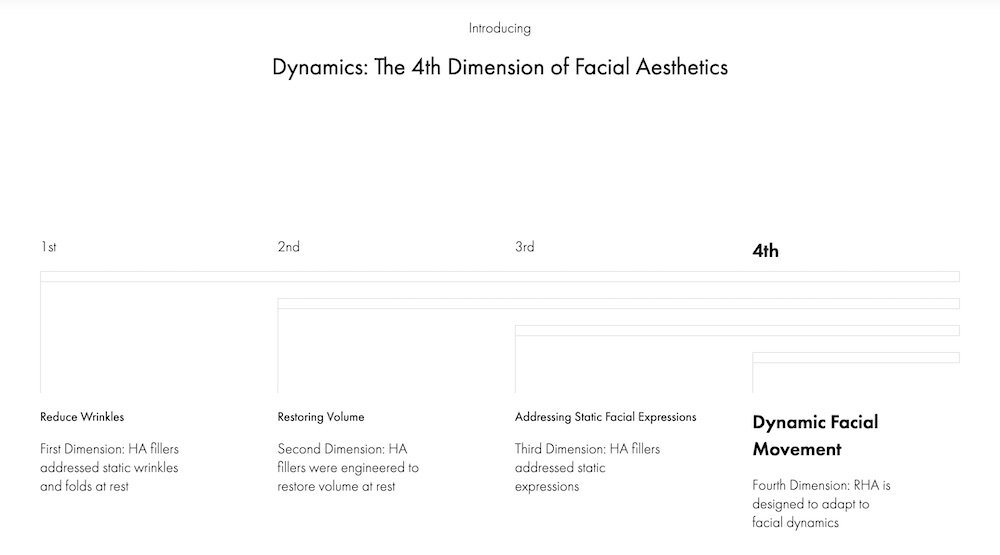
Beauty is Dynamic,
Not Static
The RHA® Collection brings you the fourth dimension because it is designed to adapt to facial dynamics.
RHA® has a unique combination of stretch and dynamic strength that makes it resilient enough to accompany, rather than endure, the demands of a constantly moving face.
Preserved Network Technology is the latest innovation in HA filler science
RHA® is designed to resemble natural HA
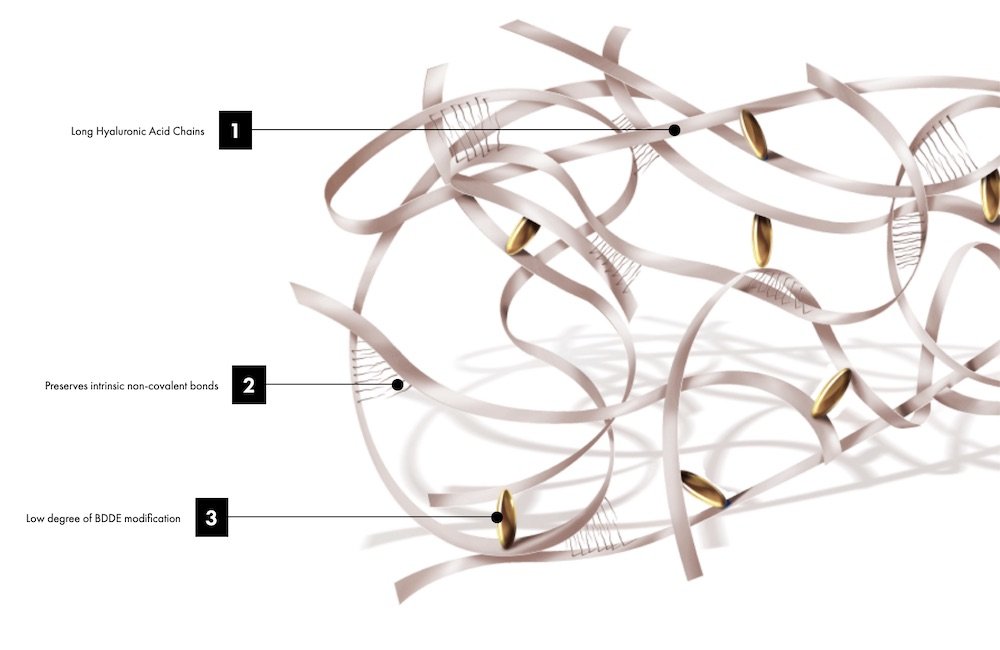
The RHA® Collection is made with a gentle manufacturing process. Reduced crosslinking allows gel to move dynamically and accompany – rather than endure – the harsh strains of facial dynamics and thus provide durable outcomes.
Closely mimics natural HA and still achieves:
- A natural look at rest and in motion
- Proven clinical effectiveness and duration up to 15 months
- No delayed onset treatment-related adverse events observed in the US clinical studies
Key Benefits
- Designed for Dynamics
The RHA® Collection is designed to adapt to the dynamic demands of the face, for a look that’s beautiful at rest and flawless in motion.
- Novel Manufacturing Approach to Resemble Natural HA
TEOXANE took a novel approach and designed a hyaluronic acid filler to more closely resemble natural HA. Preserved Network Technology preserves HA chain length and their intrinsic non-covalent bonds to more closely resemble natural HA.
PRODUCTS
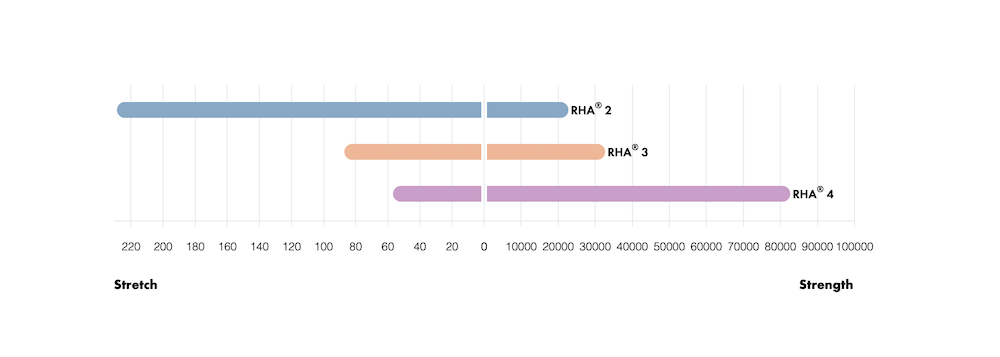
A Unique Combination of Stretch and Dynamic Strength
Resilience is the ability of a gel to maintain its integrity after stretching, bending, and being compressed, allowing the RHA® Collection to adapt to repetitive stress of facial movement.
Stretch (%/s)
Stretch is the ability of the gel to deform (extend) and adapt to dynamic facial movement. The “Stretch Score” reflects the ability of the gel to deform without disruption when subjected to continuous pressure.
Dynamic Strength (Pa2)
The “Dynamic Strength Score” characterizes the ability of a gel to maintain its physical integrity and gel characteristics over a wide range of stress or deformation values.
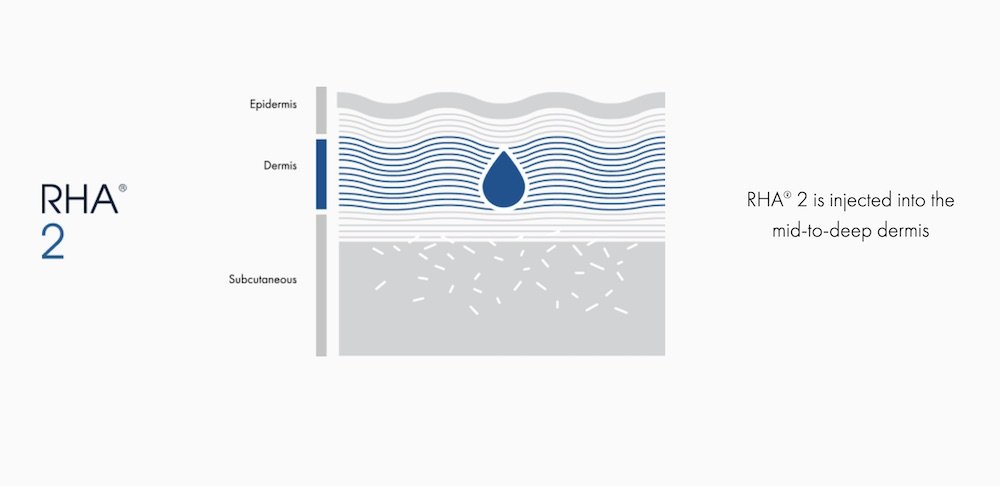
RHA® 2
For dynamic wrinkles & folds that are moderate
RHA® 2 is indicated for injection into the mid-to-deep dermis to correct moderate to severe dynamic wrinkles and folds such as nasolabial folds in adults aged 22 and older.

Before | After
She was treated with 1.3 mL of RHA® 2 in the nasolabial fold area. Images captured before and two weeks after treatment. Results may vary.

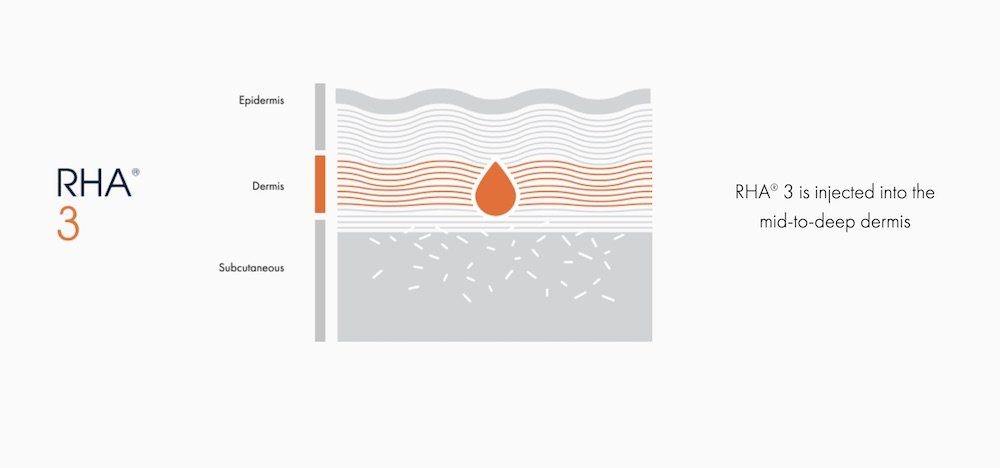
RHA® 3
For dynamic wrinkles & folds that are moderate to severe
RHA® 3 is indicated for injection into the mid-to-deep dermis to correct moderate to severe dynamic wrinkles and folds such as nasolabial folds in adults aged 22 and older.

Before | After
She was treated with 2.9 mL of RHA® 3 in the nasolabial fold area. Images captured just before and two weeks after treatment. Results may vary.

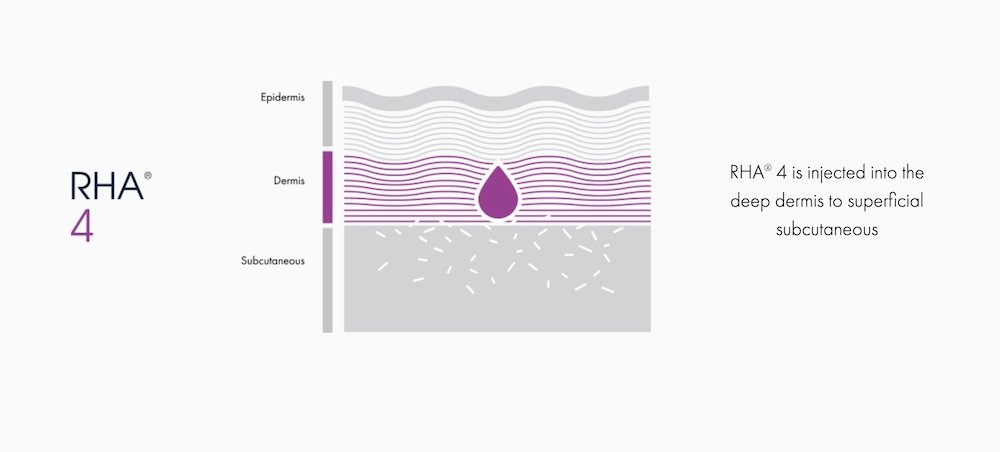
RHA® 4
For dynamic wrinkles & folds that are moderate to severe
RHA® 4 is indicated for injection into the deep dermis to superficial subcutaneous tissue to correct moderate to severe dynamic wrinkles and folds such as nasolabial folds in adults aged 22 and older.

Before | After
She was treated with 1.8 mL of RHA® 4 in the nasolabial fold area. Images captured just before and two weeks after treatment. Results may vary.

Indications and Important Safety Information
The RHA® collection of resilient hyaluronic acid (HA) fillers includes RHA® 2, RHA® 3 and RHA® 4. RHA® 2 and RHA® 3 are indicated for injection into the mid-to-deep dermis for the correction of moderate to severe dynamic facial wrinkles and folds, such as nasolabial folds; and RHA® 4 is indicated for injection in the deep dermis to superficial subcutaneous tissue for the correction of moderate to severe dynamic facial wrinkles and folds, such as nasolabial folds; in adults 22 or older.
Contraindications:
Do not use in patients who have severe allergies, marked by a history of anaphylaxis or multiple severe allergies, or in patients with a history of allergies to gram-positive bacterial proteins or local anesthetics of the amide type, such as lidocaine.
Do not use in patients with bleeding disorders.
Warnings:
Do not inject into blood vessels. Introduction of these products into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Take extra care when injecting soft-tissue fillers; for example, inject the product slowly and apply the least amount of pressure necessary. Rare, but serious, adverse events associated with the intravascular injection of soft-tissue fillers in the face have been reported and include temporary or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis, and damage to underlying facial structures. Immediately stop the injection if a patient exhibits any of the following symptoms: changes in vision, signs of a stroke, blanching of the skin, or unusual pain during or shortly after the procedure. Patients should receive prompt medical attention and, possibly, evaluation by an appropriate healthcare professional specialist should an intravascular injection occur.
Product use at specific sites in which an active inflammatory process or infection is present should be deferred until the underlying process has been controlled.
Precautions:
These products should only be used by healthcare professionals who have appropriate training, experience, and knowledge of facial anatomy.
Discuss the potential risks of soft-tissue injections with your patients prior to treatment and ensure that patients are aware of signs and symptoms of potential complications.
The safety and effectiveness for the treatment of anatomic regions other than the labeled indications have not been established in controlled U.S. clinical studies.
As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Standard precautions associated with injectable materials should be followed.
The safety for use in sites in the presence of other implants, during pregnancy, in breastfeeding females, and in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied.
Use with caution in patients on immunosuppressive therapy.
Patients who are using products that can prolong bleeding (such as thrombolytics, anticoagulants, or inhibitors of platelet aggregation) may experience increased bruising or bleeding at treatment sites.
Patients with a history of herpetic eruptions may experience reactivation of the herpes.
There is a possible risk of inflammation at the implant site if laser treatments or a chemical peel are performed after treatment.
Use as supplied. Modification or use of the product outside the Directions for Use may adversely impact the sterility, safety, homogeneity, or performance of the product.
For single patient use. Do not reuse a syringe between two treatments and/or between two patients. Do not resterilize.
Adverse Events:
The most commonly reported side effects were firmness, redness, tenderness, swelling, lumps/bumps, bruising, discoloration, pain, and itching. Most of these events were mild or moderate and resolved within 14 days.
To report an adverse reaction with any RHA®product, please call Revance at (877) 373-8669.
References
- RHA®. Directions for Use. Newark, CA: Revance Therapeutics, Inc, 2020.
- Monheit G et al. Dermatol Surg. Published online March 24, 2020. doi:10.1097/dss.0000000000002391
- Kaufman-Janette J et al. J Cosmet Dermatol. Published online August 24, 2019. doi:10.1111/jocd.13100.
- Data on file. EU Dr Brochure. Newark, CA: Revance Therapeutics, Inc, 2020.
- Micheels P et al. J Drugs Dermatol. 2017;16(2):154-161.
- de Maio M. Aesthetic Plast Surg. 2018;42(3):798-814.
- Faivre J et al. Poster presented at: 2020 IMCAS; January, 2020; Paris, France.
- Data on file. RDRE 2016 – US Products, 2016. Newark, CA: Revance Therapeutics, Inc, 2016.
- Data on file. Newark, CA: Revance Therapeutics, Inc, 2020..
- Data on File. TEO-RHA-1302 Clinical Study Report. Newark, CA: Revance Therapeutics, Inc, 2016.
- Data on File. TEO-RHA-1402 Clinical Study Report. Newark, CA: Revance Therapeutics, Inc, 2016.
