| BELOTERO BALANCE | JUVÉDERM | RADIESSE | RESTYLANE | RHA COLLECTION DERMAL FILLERS | SCULPTRA® |
Exploring Restylane treatment in Puerto Rico? Call Dr. José Raul Montes now.
Dr. Montes and his team only gives you the best results.
The Restylane family of products includes Restylane® and Perlane®. These products can be used individually to add volume and fullness to the skin to correct moderate to severe facial wrinkles and folds.
Restylane adds volume and fullness to the skin to correct moderate to severe facial wrinkles and folds, such as the lines from your nose to the corners of your mouth (nasolabial folds).
Restylane is a clear gel formulation of hyaluronic acid, specifically formulated to act like your body’s own hyaluronic acid and visibly reduce facial wrinkles and folds for a younger-looking you.
Dr. Montes provides only the best expert care for all types of beauty treatments in Puerto Rico.
1. Restylane®
2. Restylane® Lyft with Lidocaine
3. Restylane® Silk
4. Restylane® Refyne
5. Restylane® Defyne
6. Restylane® Kysse
Restylane®

Caution: Federal Law restricts this device to sale by or on the order of a physician or licensed practitioner.
Restylane is a gel of hyaluronic acid generated by Streptococcus species of bacteria, chemically crosslinked with BDDE, stabilized and suspended in phosphate buffered saline at pH=7 and concentration of 20 mg/mL.
Indication
Restylane is indicated for mid-to-deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds.
Restylane is indicated for submucosal implantation for lip augmentation in patients over the age of 21.
Contraindications
- Restylane is contraindicated for patients with severe allergies manifested by a history of anaphylaxis or history or presence of multiple severe allergies.
- Restylane contains trace amounts of gram positive bacterial proteins, and is contraindicated for patients with a history of allergies to such material.
- Restylane is contraindicated for patients with bleeding disorders.
- Restylane is contraindicated for implantation in anatomical spaces other than the dermis or submucosal implantation for lip augmentation.
Warnings
- Defer use of Restylane at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present until the process has been controlled.
- Injection site reactions (such as swelling, redness, tenderness, pain, bruising or itching) to Restylane have been observed as consisting mainly of short-term minor or moderate inflammatory symptoms starting early after treatment and with less than 7 days duration in the nasolabial folds and less than 14 days duration in the lips. Rare post-market reports of immediate post-injection reactions included extreme swelling of lips, the whole face and symptoms of hypersensitivity such as anaphylactic shock.
- Introduction of product into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Take extra care when injecting soft tissue fillers, for example inject the product slowly and apply the least amount of pressure necessary. Rare but serious adverse events associated with the intravascular injection of soft tissue fillers in the face have been reported and include temporary or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage, leading to stroke, skin necrosis, and damage to underlying facial 2 (30) structures. Immediately stop the injection if a patient exhibits any of the following symptoms, including changes in vision, signs of a stroke, blanching of the skin, or unusual pain during or shortly after the procedure. Patients should receive prompt medical attention and possibly evaluation by an appropriate health care practitioner specialist should an intravascular injection occur.
- Restylane must not be implanted into blood vessels. Localized superficial necrosis and scarring may occur after injection in or near vessels, such as in the lips, nose, or glabellar area. It is thought to result from the injury, obstruction, or compromise of blood vessels.
- Delayed onset inflammatory papules have been reported following the use of dermal fillers. Inflammatory papules that may occur rarely should be considered and treated as a soft tissue infection.
- Injections of greater than 1.5 mL per lip (upper or lower) per treatment session significantly increases the occurrence of the total of moderate and severe injection site reactions. If a volume of more than 3 mL is needed to achieve optimal correction, a follow-up treatment session is recommended.
- As with all dermal filler procedures, Restylane should not be used in vascular rich areas. Use of similar products in these areas, such as glabella and nose, has been complicated by unintentional intravascular injection resulting in embolization and symptoms consistent with ocular vessel occlusion, such as blindness. For additional information please see the Post-Marketing Surveillance in Adverse Events.
- In a meta-analysis of all Restylane Premarket Approval Studies (that included 42 patients under the age of 36 and 820 patients over the age of 35), the incidence of swelling was higher in younger patients (28%) compared to older patients (18%) and incidence of contusion was higher in older patients (28%) compared to younger patients (14%). The majority of these events were mild in severity.
Restylane® Lyft with Lidocaine

Help complete your aesthetic look with natural-looking volume in your cheeks and hands for a smoother, fuller appearance.1 Restylane® Lyft is a safe, effective and dissolvable HA filler.3 Treatment can be administered in your hands and face at the same appointment — and can result in a more youthful-looking appearance.1
WHAT IS Restylane Lyft?
Over time, natural fat begins to deteriorate, resulting in sagging skin, more prominent wrinkles and a reduction of volume. Restylane Lyft, formerly known as Perlane-L®, is an injectable hyaluronic acid gel used to correct volume loss and treat wrinkles in the face and hands. Through nonsurgical injections, Restylane Lyft is specifically designed to add fullness to the cheeks, midface area, and the back of the hands to help complete your aesthetic look — for face and hands.1
Restylane® Lyft with Lidocaine is a clear injectable gel composed of hyaluronic acid, a natural substance that already exists in the body. Restylane® Lyft with Lidocaine is non animal-based and free from animal protein. Allergy pretesting is not necessary. Restylane® Lyft with Lidocaine also contains 0.3% lidocaine. The lidocaine in Restylane® Lyft with Lidocaine has been added to reduce the discomfort associated with the treatment.
Restylane Lyft for Face
In a clinical trial, subjects treated with Restylane Lyft reported improvement with the appearance of the midface area following treatments.1†
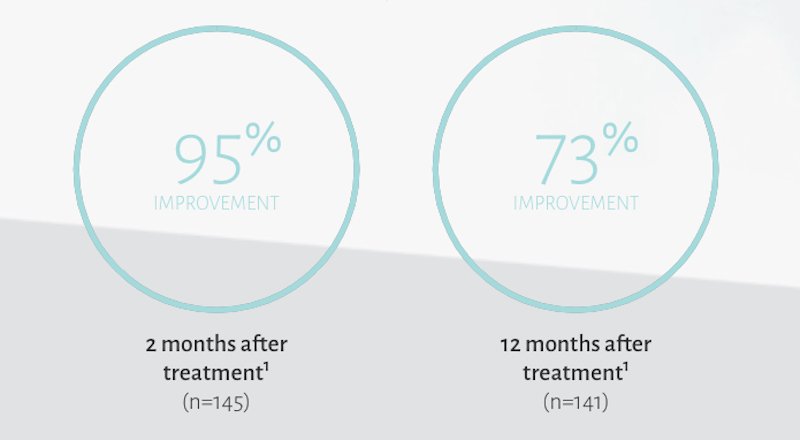
†Improvement was defined as as a score of 1 (improved) or better on the Global Aesthetic Improvment Scale at the time point of interest.
Restylane Lyft for Hands
In a clinical trial, subjects treated with Restylane Lyft demonstrated improvement‡ with the appearance of hand volume following treatments.1
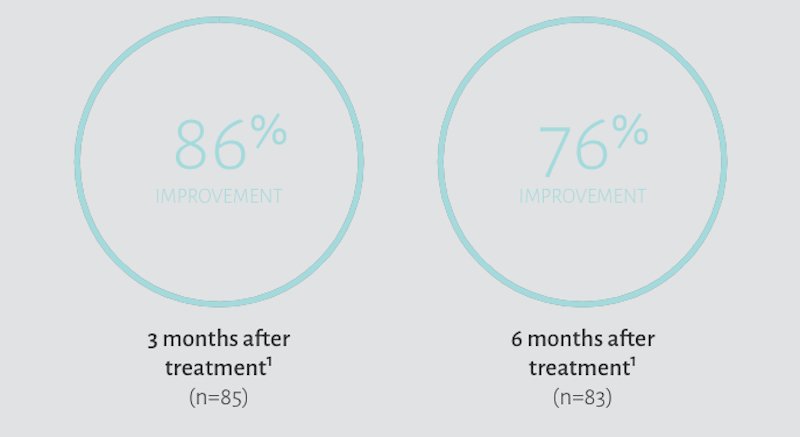
‡Improvement was defined as subjects with Merz Hand Grading Scale (MHGS) score of ≥1 grade from baseline as assessed by blinded evaluators.
How does Restylane® Lyft with Lidocaine work?
Restylane® Lyft with Lidocaine is injected into the skin with an ultrafine needle to plump the skin to smooth away wrinkles and folds such as the lines from your nose to the corners of your mouth and is injected with an ultrafine needle or a small, blunt-tipped cannula to increase the fullness of your 2 (13) cheeks. Restylane Lyft with Lidocaine may also be injected, using an ultrafine needle under the skin to address volume deficiency in the back of the hand.
Why is there lidocaine in Restylane® Lyft with Lidocaine?
The lidocaine in Restylane® Lyft with Lidocaine reduces pain and discomfort during and after injection.
The effectiveness of the lidocaine was studied in a split-face clinical study of 60 patients. Each patient received Restylane® Lyft without Lidocaine on one side of the face and Restylane® Lyft with Lidocaine on the other side of the face for the treatment of lines around the nose and mouth. Restylane® Lyft with Lidocaine significantly reduced the pain of the injection.
- For the side of the face treated with Restylane® Lyft without Lidocaine, patients rated their pain as about 47 on a scale of 0 to 100 after injection.
- For the side of the face treated with Restylane® Lyft with Lidocaine, patients rated their pain as about 15 on the same scale.
- Pain relief from the lidocaine in Restylane® Lyft with Lidocaine lasted up to 60 minutes after treatment.
How long does Restylane® Lyft with Lidocaine last?
When Restylane® Lyft with Lidocaine is used for the treatment of wrinkles and folds such as the lines from your nose to the corners of your mouth, the benefits generally last about 6 months as the filler gradually disappears from the body.
When Restylane® Lyft with Lidocaine is used to increase the fullness of your cheeks, the benefits generally last between two and twelve months as the filler gradually disappears from the body. The graph below compares patients who were treated with Restylane Lyft with Lidocaine using a needle and patients who had no treatment, who showed a positive treatment response at 2, 4, 6, 8, 10 and 12 months, as assessed by blinded evaluators (e.g., physicians not aware of the patient treatment assignment).
When Restylane® Lyft with Lidocaine is used to increase fullness in the back of the hand, the benefits usually lasts 6 months as the filler gradually disappears from the body.
What are some potential risks that I may encounter?
Other potential risks may arise from an injection of a dermal filler such as Restylane® Lyft with Lidocaine including the following:
Infection – Facial skin injections, including those with Restylane® Lyft with Lidocaine, are associated with a risk of infection. As with any infection, there may be a need for your doctor to prescribe antibiotics. Though rare, a skin infection could appear as small, swollen (or red) bumps (inflammatory papules). If an infection worsens over time, you should contact your doctor for further treatment.
Scarring – While it is very rare, scarring may occur with an injection procedure. In clinical trials, excessive scarring (keloids) was not observed in any of the patients receiving Restylane® Lyft with Lidocaine.
Change in skin tone – If you are African American, you may have a higher risk of darkening of the skin tone in the treated area (hyperpigmentation). This may take several weeks to disappear. In clinical trials, this change in skin tone did not occur with injection of Restylane® Lyft with Lidocaine into the cheeks.
Cold sores – If you have had cold sores in the past, there is a risk that they will return as a result of facial injections.
Age and pregnancy – If you are under 18 or over 65 years of age, are pregnant or trying to become pregnant, or are nursing, you should know that the safety and effectiveness of Restylane® Lyft with Lidocaine has not been established in patients like yourself. In addition, for treatment of the cheeks, Restylane® Lyft with Lidocaine has not been evaluated in patients younger than 22 years of age.
Vision changes – Rarely, vision abnormalities have been reported after treatment with Restylane® Lyft with Lidocaine.
Use of other skin therapies – The safety of Restylane® Lyft with Lidocaine used with other skin therapies such as laser, mechanical or chemical peeling, and hair removal has not been established. The use of Restylane® Lyft with Lidocaine with these skin therapies may lead to other side effects such as inflammation.
Ultraviolet (UV) exposure – You should avoid exposing the area(s) treated with Restylane® Lyft with Lidocaine to excessive sun, UV lamps or indoor tanning beds/booths, and extreme heat and cold until any redness or swelling has disappeared. Exposure to UV sources may result in irritation at the site of treatment.
Thumb Function – You may experience a slight decrease in the flexibility of your thumb after treatment with Restylane® Lyft with Lidocaine in the back of your hand.
If you have any additional questions or concerns about these potential risks, please discuss them with
your doctor.
NATURAL-LOOKING before + after
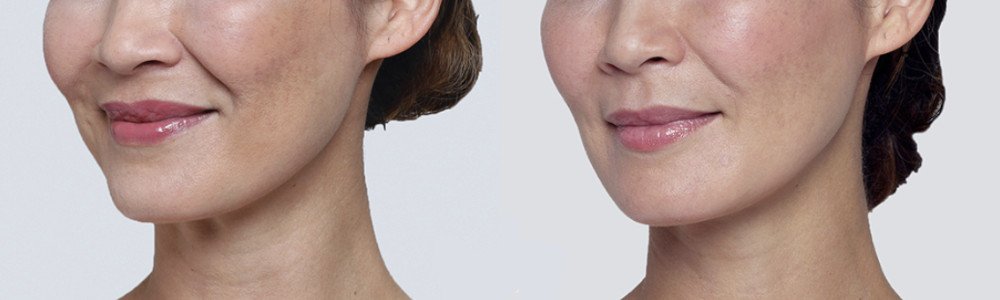


References:
- Restylane Lyft. Instructions for Use. Galderma Laboratories, L.P., 2018.
- U.S. Food & Drug Administration. Medical Devices, Restylane Lyft with Lidocaine – P040024/S099. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm609592.htm. Accessed on May 18, 2018.
- Data on file. Fort Worth, TX: Galderma Laboratories, L.P., 2018.
- Data on file. Restylane Survey Demographic Report (Wakefield Research), March 2018.
- Real-time data. Real Self website.
Restylane® Silk
Injectable Gel with 0.3% Lidocaine

WHAT IS Restylane Silk?
The lips and the lines around the mouth, like other areas of the face, show signs of aging as you get older. This often results in lip thinning, lost shape and an increase in vertical lines above the lip. Restylane Silk is designed specifically to provide natural-looking results in these particular areas by using smaller, smoother hyaluronic acid particles than those used in other Restylane® products.
WHERE DOES RESTYLANE SILK WORK?
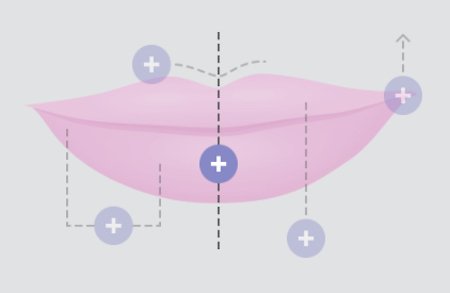
FULLNESS IN THE CENTER
Lips look most beautiful when the fullness in the center tapers off at the corners.
DEFINED “CUPID’S BOW”
The “cupid’s bow” of the upper lip is most attractive when it is well defined and when the two sides look the same.
SIDE-TO-SIDE SYMMETRY
From one side to the other, lips should be symmetrical for a pleasing appearance.
PROJECTION ON PROFILE
When viewed from the side, the upper lip should project slightly beyond the lower lip.
LIPS-TO-EYES RELATIONSHIP
A line drawn upward from the corner of the lips should hit the midpoint of the pupil of the eye.
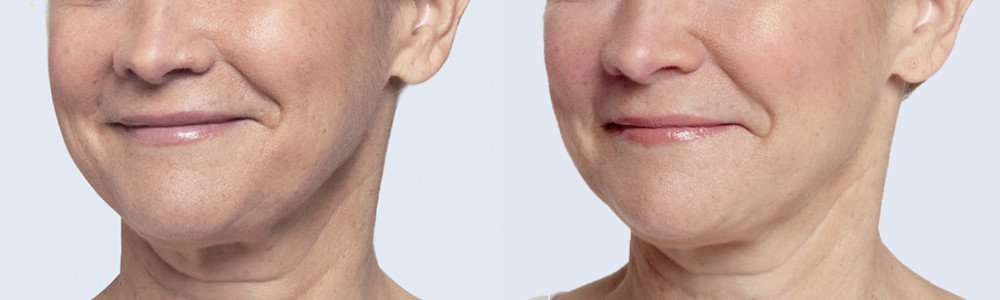
NATURAL-LOOKING before + after
Treated with 2 mL of Restylane Silk in the lips and perioral (lip) lines around the mouth, and 1 mL Restylane-L in nasolabial folds. 4 weeks after treatment.
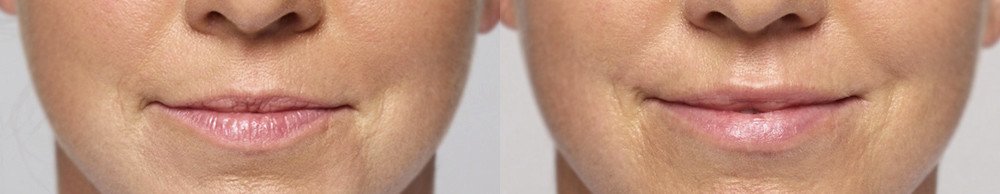
Treated with 2 mL of Restylane Silk in the lips and 1 mL of Restylane in the nasolabial folds and marionette lines. 4 weeks after treatment.
References:
- Real-time data. Real Self website.
- Restylane Silk. Instructions for Use. Galderma Laboratories, L.P., 2017.
Restylane® Refyne

WHAT IS Restylane Refyne?
As skin loses elasticity with age, the lines that run from the sides of your nose toward the corners of your mouth (nasolabial folds) and the lines that run from the corners of your mouth toward the chin (marionette lines) appear more noticeable. Restylane Refyne is a specifically formulated hyaluronic acid dermal filler made with XpresHAn TechnologyTM that helps correct these lines for a more natural look to your face.3,4 The unique cross-linking of the gel in Restylane Refyne is also designed to help support your natural expression—for real-life results that help maintain natural movement when you’re smiling, frowning and even puckering up.1,2

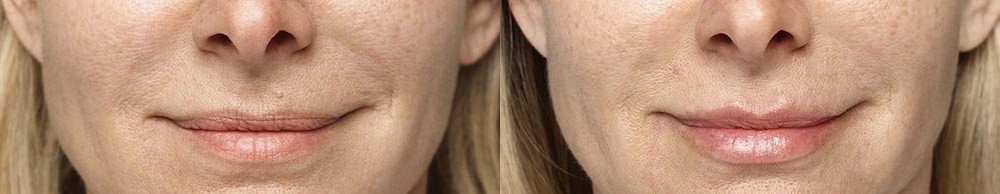
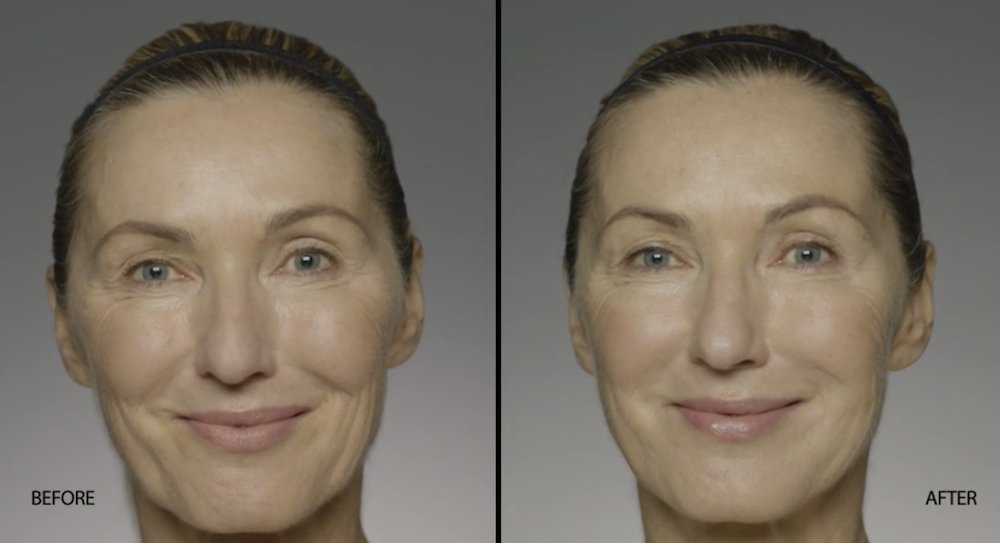
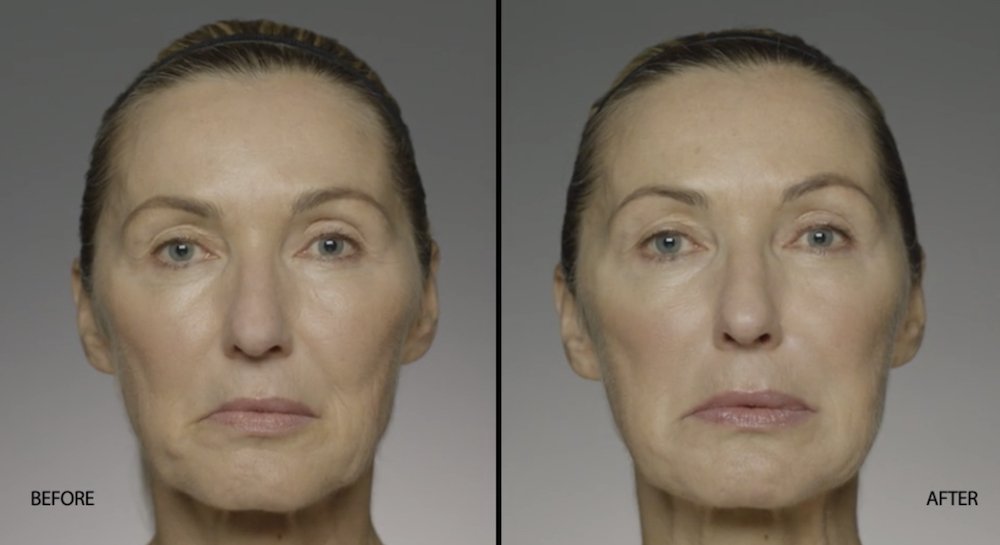
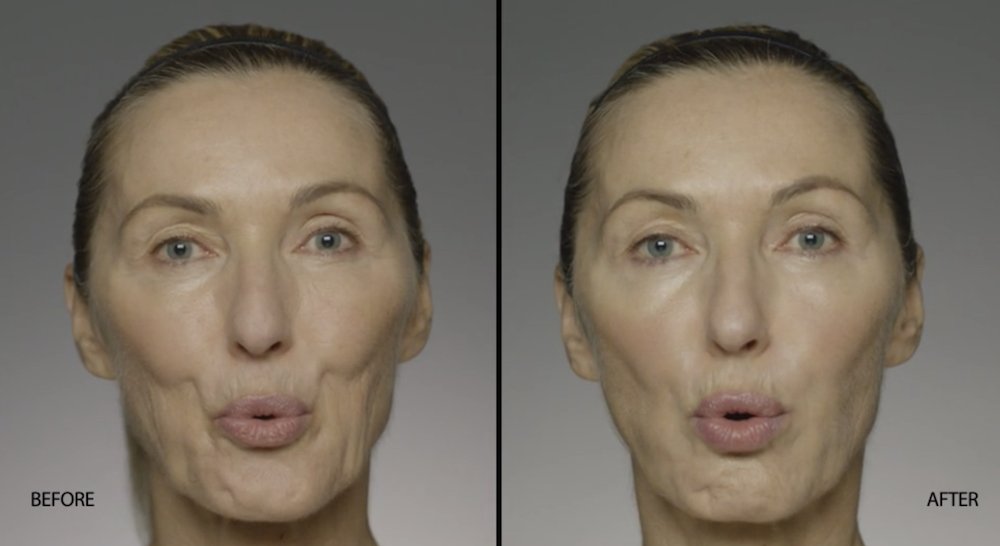
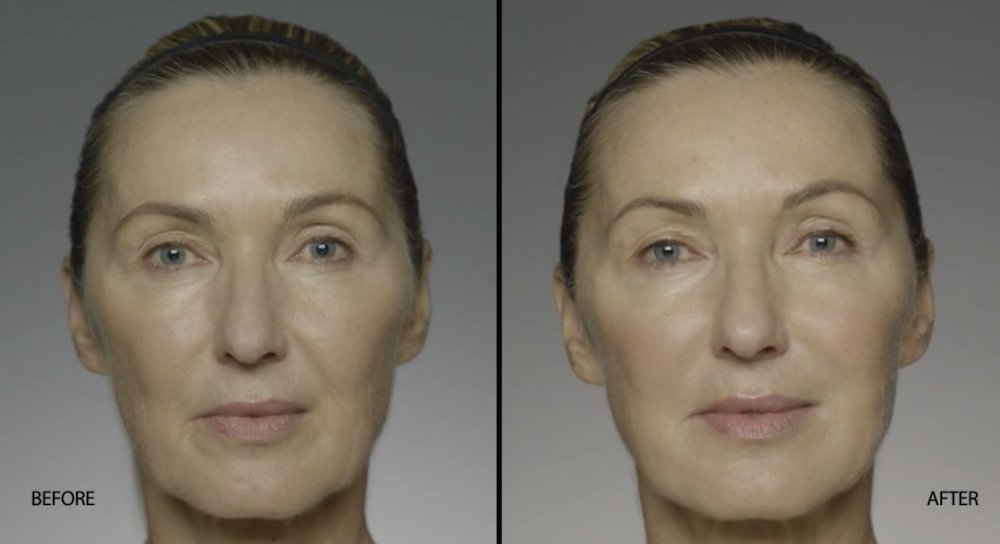
NATURAL-LOOKING before + after
These images are unretouched photographs of actual patients who have used Restylane Refyne to achieve natural-looking results.
Treated with 1.5 mL Restylane Refyne to nasolabial folds and 1.0 mL Restylane Refyne to marionette lines. 2 weeks after treatment.
References:
- Data on file. 05DF1502 Clinical Study Report. Fort Worth, TX: Galderma Laboratories, L.P., 2016.
- Data on file. MA-32418 Study Report. Fort Worth, TX: Galderma Laboratories, L.P., 2016.
- Restylane Refyne. Instructions for Use. Galderma Laboratories, L.P., 2016.
- Segura S, Anthonioz L, Fuchez F, Herbage B. A complete range of hyaluronic acid filler with distinctive physical properties specifically designed for optimal tissue adaptations. J Drugs Dermatol. 2012;11(1 Suppl):s5-s8.
- Real-time data. Real Self website.
Restylane® Defyne


WHAT IS Restylane Defyne?
As we age, our skin loses elasticity and the lines that run from the sides of the nose toward the corners of the mouth (nasolabial folds) and the lines that run from the corners of your mouth toward the chin (marionette lines) become more pronounced. Restylane Defyne is crafted with XpresHAn TechnologyTM to correct these deepened lines.3 The unique cross-linking of the gel in Restylane Defyne is also designed to help support your facial expressions—for natural-looking results that help maintain your natural movement.1

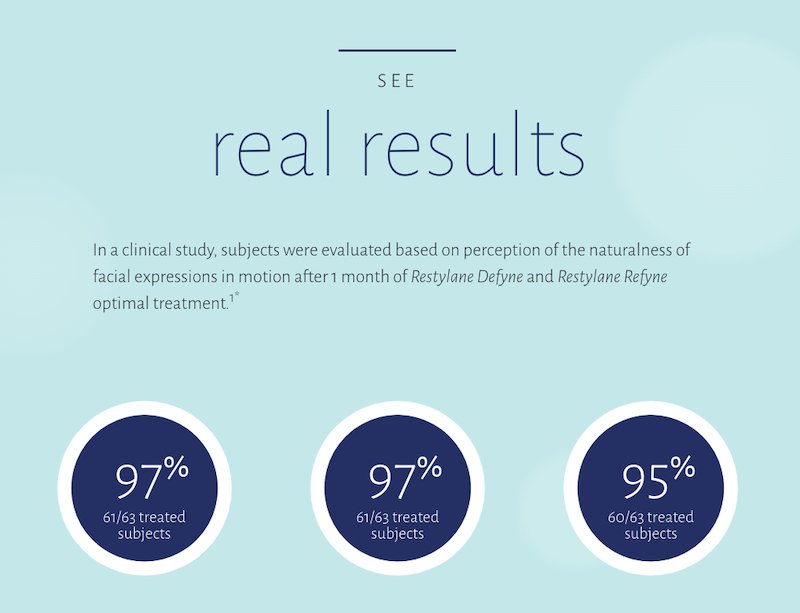
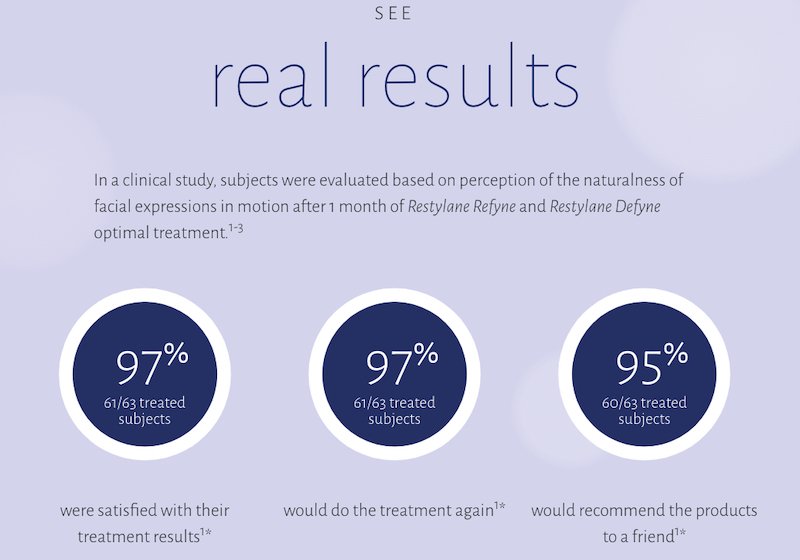
Restylane Defyne and Restylane Refyne are the only fillers on the market that have been shown in a clinical study to help maintain movement and expressions.1
*This was a multicenter, noncomparative trial of subjects aged 35-65 years (N=63). Subjects were evaluated based on the perception of the naturalness of facial expressions in motion after 1 month of Restylane Defyne and Restylane Refyne optimal treatment. Pooled results for both Restylane Refyne and Restylane Defyne subjects.1
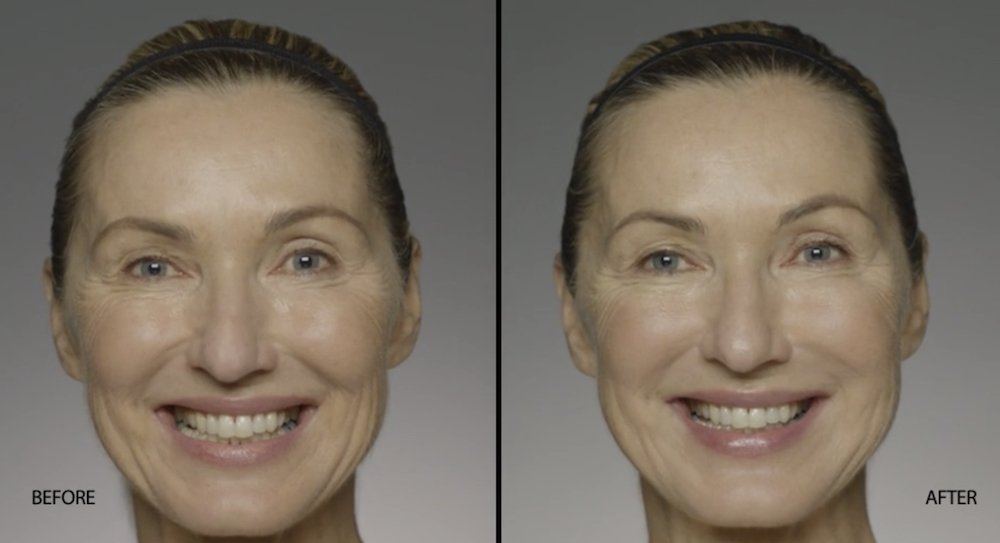
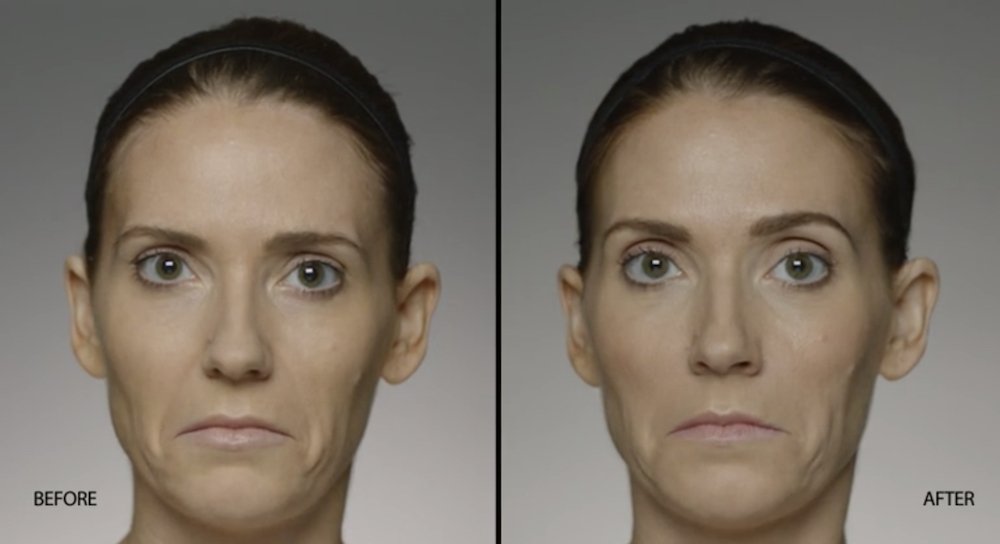
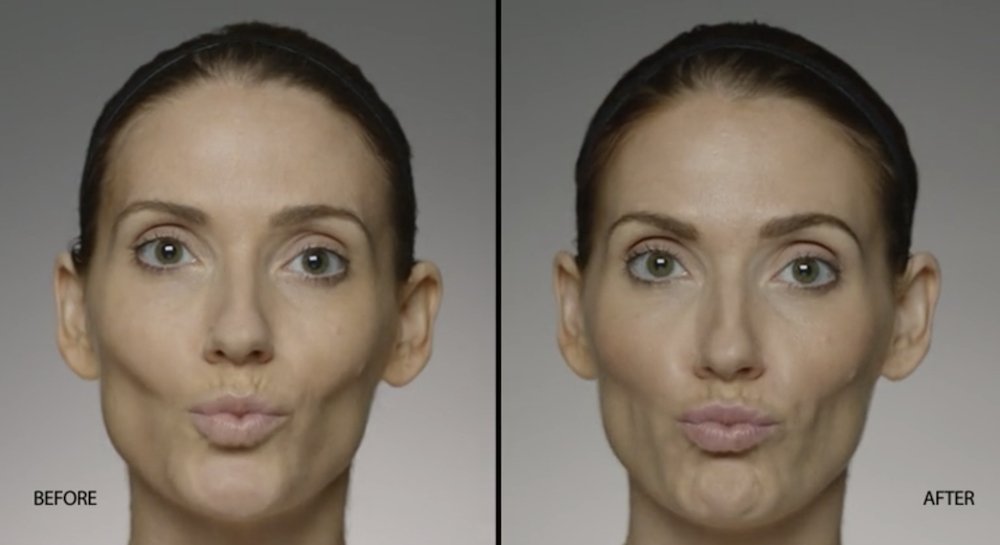
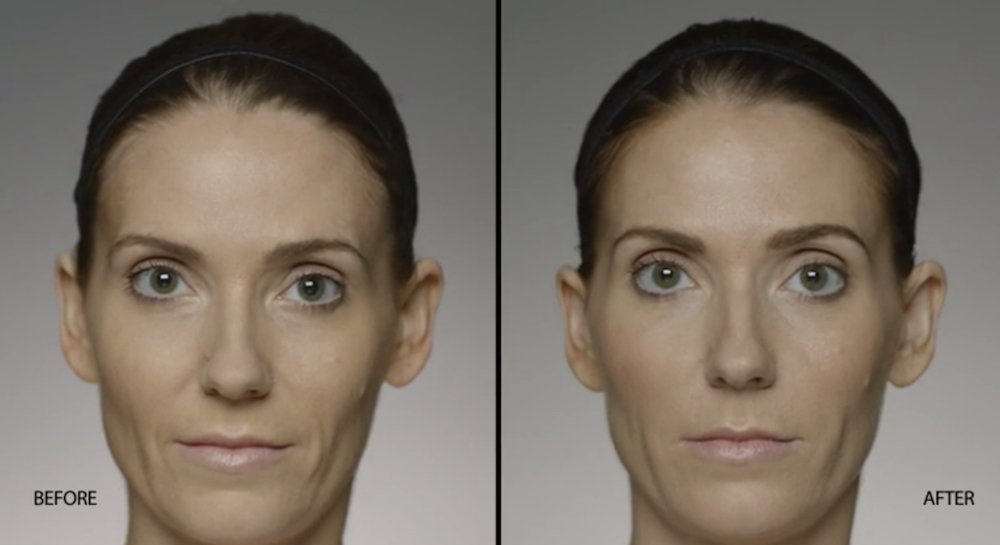
NATURAL-LOOKING before + after
These images are unretouched photographs of actual patients who have used Restylane Defyne to achieve natural-looking results.


References:
- Data on file. 05DF1502 Clinical Study Report. Fort Worth, TX: Galderma Laboratories, L.P., 2016.
- Restylane Defyne. Instructions for Use. Galderma Laboratories, L.P., 2016.
- Segura S, Anthonioz L, Fuchez F, Herbage B. A complete range of hyaluronic acid filler with distinctive physical properties specifically designed for optimal tissue adaptations. J Drugs Dermatol. 2012;11(1 Suppl):s5-s8.
- Real-time data. Real Self website.
Restylane® Kysse
What is Restylane Kysse?
Restylane Kysse is a clear injectable gel composed of hyaluronic acid (HA). HA is a naturally occurring sugar, found in the human body.
Restylane Kysse is crosslinked with BDDE, an ingredient that helps form a network of HA to provide a gel filler that lasts longer. HA fillers, including Restylane Kysse, contains HA that has been modified to last longer in the body than the naturally occurring HA. Restylane Kysse contains 0.3 % lidocaine to reduce pain during injection.
What is Restylane Kysse for?
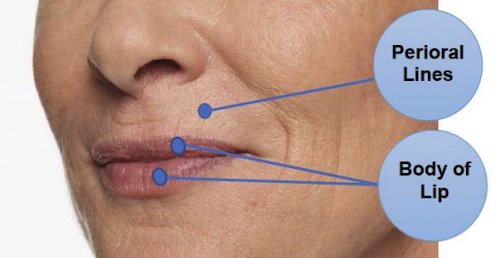
Restylane Kysse is injected into the lips and the lines around the upper lip to temporarily add fullness to the lips and help smooth the wrinkles around the upper lip (perioral lines) in patients over the age of 21.
How is Restylane Kysse used?
Restylane Kysse is injected with an ultrafine needle into your lips to add fullness. It can also be injected into the skin above your lips to help smooth the wrinkles around your upper lip (perioral lines). The picture below shows the treatment areas for Restylane Kysse.
What will Restylane Kysse do for me?
Restylane Kysse will temporarily add volume to the lips and help smooth the wrinkles above your mouth.
Restylane Kysse was studied in many subjects like yourself to determine if it was effective and to ensure that it is safe for use.
The study doctors reported:
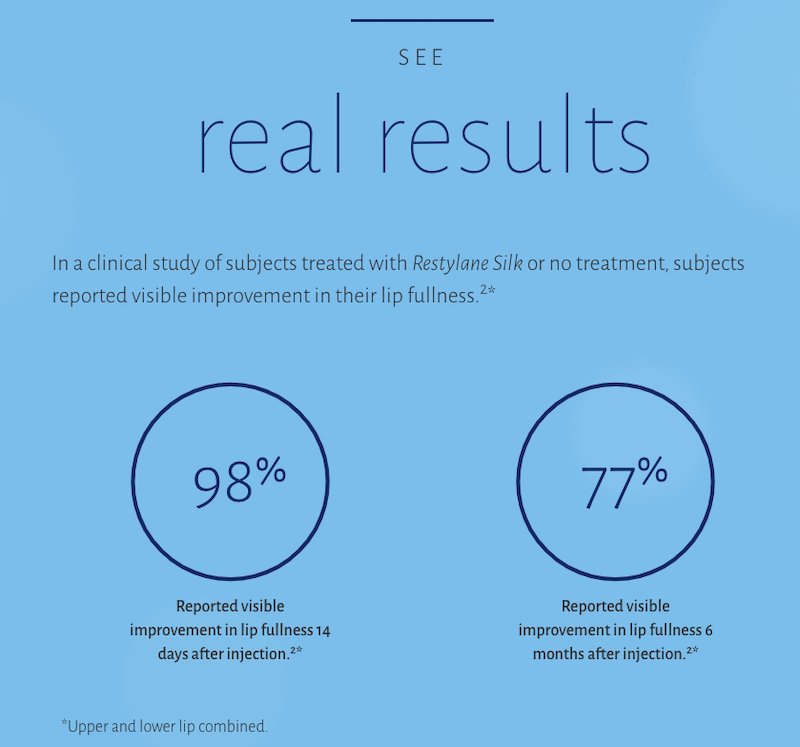
- 77% (129/168) of subjects had at least a 1-point improvement in lip fullness 6 months after treatment
- 60% (101/169) of subjects had at least a 1-point improvement in lip fullness 12 months after treatment
The subjects reported:
- 88% (148/169) rated an improvement in the appearance 6 months after treatment
- 78% (132/169) rated an improvement in the appearance 12 months after treatment
Are there any reasons why I should not receive Restylane Kysse?
Your doctor will ask about your medical history to determine if Restylane Kysse is right for you. Patients with any of these conditions should not use Restylane Kysse:
- Highly allergic (for example: a history of severe allergic reaction or multiple severe allergies). Use may result in an allergic reaction.
- If you have previous experience with allergic reactions to HA fillers. Use may result in an allergic reaction.
- Allergic to anesthetics, such as lidocaine. Use may result in an allergic reaction.
NATURAL-LOOKING before + after
Cassandra, 27 years
After 1 month*
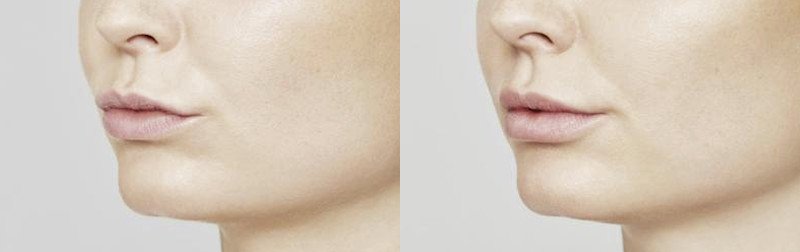
Maria, 53 years
After 1 month*

Important safety information
The Restylane family of products are indicated for patients over the age of 21, and includes Restylane®, Restylane-L®, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Refyne, Restylane® Defyne and Restylane® Kysse.
APPROVED USES
Restylane® and Restylane-L® are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. Restylane® and Restylane-L® are also indicated for injection into the lips.
Restylane® Lyft with Lidocaine is for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation and for the correction of age-related midface contour deficiencies. Restylane® Lyft with Lidocaine is also indicated for injection into the dorsal hand to correct volume loss.
Restylane® Silk is for lip augmentation and for correction of perioral wrinkles.
Restylane® Kysse is for lip augmentation and for correction of upper perioral wrinkles.
Restylane® Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds.
Restylane® Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds.
Do not use if you have severe allergies with a history of severe reactions (anaphylaxis), are allergic to lidocaine or gram-positive bacterial proteins used to make hyaluronic acid, prone to bleeding, or have a bleeding disorder. The safety of use while pregnant or breastfeeding has not been studied. Tell your doctor if you have a history of scarring or pigmentation disorders as these side effects can occur with hyaluronic acid fillers. Tell your doctor if you are planning other cosmetic treatments (i.e., lasers and chemical peels) as there is a possible risk of inflammation at the injection site.
Tell your doctor if you’re taking medications that lower your body’s immune response or affect bleeding, such as aspirin or warfarin, as these medications may increase the risk of bruising or bleeding at the gel injection site. Using these products on gel injection sites with skin sores, pimples, rashes, hives, cysts, or infections should be postponed until healing is complete.
The most common side effects are swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site, and impaired hand function. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis at the injection site. The risk of unintentional injection into a blood vessel is small but can occur and could result in serious complications, which may be permanent including, vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. As with all skin injection procedures, there is a risk of infection.
To report a side effect with any Restylane product, please call Galderma Laboratories, L.P at 1-855-425-8722.
To learn more about serious but rare side effects and full Important Safety Information, visit www.RestylaneUSA.com.
